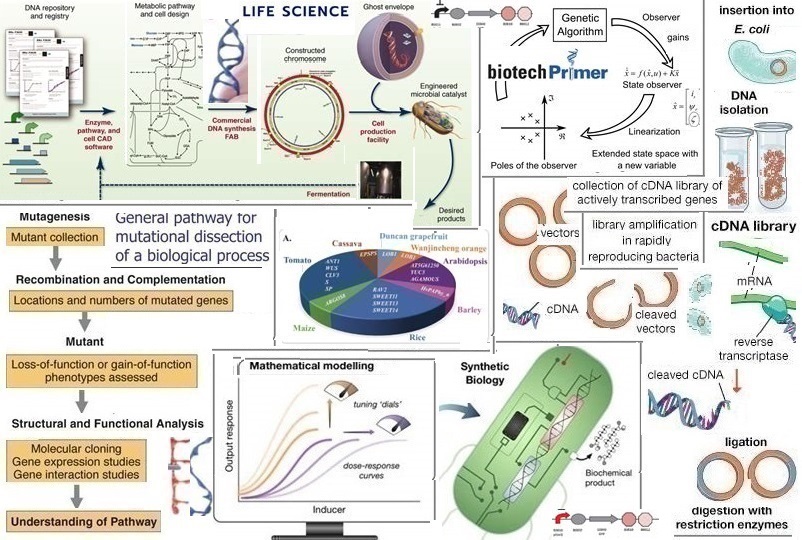
Genetic engineering (manipulation or modification) is the process of using laboratory technologies such as recombinant DNA technology (which developed from fundamental research in microbial genetics) or other nucleic acid molecules to modify an organism’s DNA genotype to achieve significant qualities. With the increase in influenza, Middle East respiratory syndrome (MERS), and SARS, the United States Government banned all federal funding for gain-of-function studies during October 2013. There are several unanswered moral concerns about the technology. In the debate surrounding SARS -CoV-2, the virus that causes COVID -19, gain of function does not play a crucial role in a virus that is more infectious between humans or that has a higher fatality rate in humans. With the success of its COVID-19 mRNA vaccine, BioNTech is determined to develop vaccines for other infectious diseases such as malaria and tuberculosis.
To fully understand the potential risks that accompany novel viruses, there is a lack of research and experiments that can be classified as ‘gain of function’ research (GOFR) that include the use of pathogens. DNA is a polymer that consist of two polynucleotide chains that spiral around each other to produce a double helix. The polymer contains genetic ‘guidelines’ for identified organisms (as well as several viruses) to develop, function, and reproduce. Genes consist of DNA (the natural component of heritage) in humans. Some genes are ‘instruction genes’ that have the function of generating molecules known as proteins while others are excluded from encrypting for proteins. A marker is a section of DNA where ‘dictation’ of a gene originates. Markers are important aspects of expression vectors because they monitor the bonding of RNA polymerase to DNA. This is a vital phase in any DNA experiment since this is where RNA polymerase alters DNA to mRNA and finally into a suitable protein. GOF systems are based on the addition of compatible elements to detect genes that alter genome development. P-elements were discovered while in the process of researching a genetic syndrome known as ‘hybrid dysgenesis.’ The P-element transforms an enzyme defined as P transposase. Various genome P-element insertion studies have been completed in research laboratories; based on its inclusion points in the genome, the P-element can disrupt individual genes.
Transcription (expression) factors are proteins that regulate the transformation of genes which is their replication into RNA before the final conversion into protein. The human body has many transcription factors to ensure that accurate genes are extracted to the correct cells in the body. GATA binding protein 3 (GATA3) is not only a transcription factor that contributes to human growth but the GATA3 gene encodes a protein that belongs to the GATA family of transcription factors; the protein regulates T-cell development. GATA3 is a well-preserved transcription factor transferred into various tissues, including the mammary glands. Cancer is a disease caused by genetic mutations; delineations of gene transcription demonstrated that GATA-3 is among the repeatedly mutated genes in breast cancer. Mutation patterns indicate a gene’s function as either tumour promotive or tumour suppressive. In the inadequately researched GATA3 gene two different groups of mutations can progress to gain- or loss. The GATA3 gene is excluded from this principle. Johnson and Johnson’s Covid-19 vaccine was created via GOF methodology. A virus known as an adenovirus (the ‘common cold virus’ usually responsible for flu- like symptoms) was used for a fraction of the SARS-CoV-2 genetic material. The term ‘gain of function’ includes a wide field. Functioning genomes can be made from synthetic chemicals which allows genetic engineers to create artificial genomes as an alternative to modifying natural ones. According to the Boston University there are reasons for concern that factors such as urbanisation, ever-increasing greenhouse gas emissions, and non-biodegradable waste material will drastically increase the potential of infectious diseases and future pandemics.
After the Covid-19 pandemic the federal Government created the National Science Advisory Board for Biosecurity (NSABB) to assess policy structures for the biosecurity regulations of life sciences research. NSABB (managed by NIH’s Office of Science Policy) addresses issues involving biosecurity and dual-use research. The Advisory Board is a committee of specialists that supports the Secretary of the United States Department of Health and Human Services by recommending policies on issues such as how to prevent published research in biotechnology from promoting terrorism without reducing scientific progress. On 27 January 2023, the Board assembled per virtual platform to discuss the draft report of the NSABB Working Groups to review and evaluate the United States Government Potential Pandemic Pathogen Care and Oversight (PC3O) and Dual Use Research of Concern (DURC) Policies. The NSABB has more than twenty voting members with a wide selection of expertise such as biodefense, biosafety, infectious diseases, microbiology, molecular biology, plant health, public health, veterinary medicine, scientific publishing, national security, and law enforcement.
Some methods of gain-of-function research involves specific agent pathogens which transfer biosafety risks known as dual use research of concern (DURC). The United States and European Union regulations dictate that one or more unaffiliated members of the public must be ‘active participants’ in the supervision procedures. To reduce any risks while approving GOF research, several governments have instructed further control of DURC experiments under the supervision of administrations (known as institutional ‘DURC’ committees) and government agencies (such as the NIH’s DNA advisory committee). A similar example is the European Union’s Dual Use Coordination Group (DUCG). Gain-of-function occurs when there is an alteration to any organism through a process that either causes it to gain a new function or improve an existing function. Several GOF experiments have raised alarm because of the risk to humans via the infectious virus used during the experiments. Gain-of-function refers to ‘research which could enable a pandemic-potential pathogen to replicate quickly or cause more harm to humans or other closely related mammals.’ Nobody knew that a virus caused the 1918 flu pandemic, much less did they understand that animals could be carriers of human illnesses.
Nowadays, virus ecosystem observation and research have become an indispensable obligation of public health. Until 1999, it was believed that the influenza B virus (which circulated among humans and triggered seasonal epidemics) could only infect humans. However, recent data verified that seals can also be infected with the influenza B virus. Infecting rabbits with a mutation of influenza B in a controlled laboratory scenario would classify as a gain-of-function experiment as the function did not previously exist in the virus. This type of experiment can reveal which fragments of the virus’s genome resemble the species that it is able to infect, allowing the production of antiviral medication to block this function. Gain-of-function research (GOFR) refers to medical research that genetically modifies an organism to improve the biological functions of gene products. These advanced functions include the host range (the additional types of hosts that a micro-organism can now infect), transmissibility, and transformed pathogenesis. In virology, GOFR is the optimal method to unidentified solutions for existing and future pandemics. In the race for pandemic vaccines, the first phase in GOFR is to extract viruses from animals before genetic alteration in a laboratory (to make them more transmissible to humans); then the stages follow one another to develop a vaccine before the pandemic-causing virus progresses. GOF research is used in many other ways, but the process becomes provocative when scientists increase the risk of infectious pathogens to humans.
The scientific community has had substantial discussions about the evaluation of the advantages and disadvantages of GOFR, how to distribute GOF research, and how to engage the public in evaluations. In January 2020, NSABB re-evaluated the guidelines for GOFR and determined when the results of these experiments should be released to the public. During 2011, two groups were examining how bird flu viruses could cause pandemics in humans: one group was directed by Yoshihiro Kawaoka at the University of Wisconsin and the other group was controlled by Ron Fouchier at Erasmus University Medical Center in the Netherlands. Both groups successfully introduced the H5N1 avian influenza virus to ferrets by manually exposing the virus from one ferret to another, until it was able to eventually spread via respiratory droplets. Through replication over time into the ferrets’ lungs the bird-specific virus implemented several amino acid changes that allowed it to reproduce in the mammalian lungs (which are colder than those of birds). This minor alteration permitted the virus to spread via droplets in the air when the ferrets coughed or sneezed. Supporters of the Kawaoka and Fouchier experiments emphasised advantages such as the H5N1 virus becoming airborne in humans, the relation between the transmissibility of avian viruses and fatality (while the virus had become more infectious, it converted to a less fatal infection), and the production of vaccines that targeted the amino acid changes. Many opponents of the research (including members of Congress) responded with apprehension to the publication of the experiments. There were queries from scientists including Marc Lipsitch of the T. H. Chan School of Public Health at Harvard University regarding the risks of the research. According to a new study, these 2011 avian flu virus experiments caused more than one-hundred-and-fifty seal deaths in New England. The study also confirmed that the virus can easily spread through respiratory drops, infect other mammals, and pose a threat to humans.
In May 2013, a group directed by Hualan Chen, director of China’s National Avian Influenza Reference Laboratory, published many of their experiments at the BSL3+ laboratory of the Harbin Veterinary Research Institute in which they experimented what would happen if a 2009 H1N1 circulating in humans infected the same cell as an avian influenza H5N1. Hualan Chen’s group carried out the experiments before a ‘research gap’ on H5N1 experiments could be approved by the larger virologist community. The purpose of the experiments were to determine that genes (if re-assorted in such a dual-infection setup in the wild) would allow easier transmission of the H5N1 virus in mammals (particularly in guinea pigs as a representative animal for rodent species) and verifying that specific agricultural situations risk the crossover of H5N1 into mammals. As in the Fouchier and Kawaoka experiments, the viruses in the May 2013 study were also less fatal after modification. Critics of the 2013 Chen group study (including Simon Wain-Hobson of the Pasteur Institute and former Royal Society President Robert May) disapproved of the experiment as not only pointless, but very dangerous research, summarising Chen’s research as intolerable negligence that raises alarm about the biosafety of the laboratory. Jeremy Farrar, director of the Oxford University Clinical Research Unit in Ho Chi Minh City, defined the work as ‘outstanding’ but expressed his concerns as a ‘very real threat’ and ‘continued circulation of H5N1 strains in Asia and Egypt.’ All research subsidised by the NIH is scrutinised by an additional gain-of-function assessment.
The Cambridge Working Group published a Consensus Statement transcribed by eighteen founding members. Since its first publication, more than three hundred academics, physicians, and scientists have added their signatures. The statement demands that all research including potential pandemic pathogens be paused until an impartial assessment of the risks has been determined. Scientists for Science (SfS) was formed by thirty-seven parties who took a different approach namely ‘biomedical research on potentially dangerous pathogens can be performed safely and is essential for a comprehensive understanding of microbial disease pathogenesis, prevention and treatment.’ Since its publication, the SfS statement has received more than two hundred signatures from academics, biosafety professionals, and scientists. W. Paul Duprex (virologist at the University of Pittsburgh) and other SfS signatories stress the fact that the pathogens are already governed by extensive regulations. They reason that the research would be beneficial when the experiments are focused on the interest of the public and to rather refine laboratory safety and supervision.
The National Research Council (NRC) is the operational branch of the United States National Academies of Sciences, Engineering, and Medicine and is managed by a governing committee of councillors from each of the three Academies. The purpose of NRC is to increase scientific research, to investigate natural phenomena, and to develop American industries by co-ordinating educational, government, industrial, and other research organisations. The NRC Research Associateship Programs (RAP) is administered by the United States Government. RAP promotes excellence in scientific and technological research by presenting senior level, graduate, and postdoctoral research opportunities at subsidised government laboratories and associate institutions.
The COVID-19 pandemic revealed the devastating effect of disease. The Biological and Toxin Weapons Convention (BTWC) was the first multi-lateral settlement to prohibit a complete category of weaponry. The treaty banned the accumulation, acquisition, development, production, transfer, and use of biological and toxic weapons (biological agents and toxins of ‘types and quantities’) that have no validation for defensive or non-violent use. Today, with more than one hundred and fifty state parties, it is the foundation of international efforts to counteract biological weapons.
Founded in 1887, The National Institutes of Health (NIH) is a United States Government research agency responsible for biomedical and public health research and is a section of the U.S. Department of Health and Human Services. Being the world’s largest medical research agency (conducting and supporting basic, clinical, and medical research), NIH has twenty-seven different Institutes and Centres which focus on improving health through the assistance of biomedical and behavioural research. The primary federal agency for research on mental disorders is the National Institute of Mental Health (NIMH). Global leadership for education, training, and research to promote the treatment and prevention of heart, lung, and blood diseases is provided by the National Heart, Lung, and Blood Institute (NHLBI). NIH has an obligation to reveal the results of NIH-funded research.
The NIH Loan Repayment Programs (LRPs) are programs established by Congress and intended to employ and sustain highly competent health specialists into biomedical and biobehavioural research professions. Reliable data management and distribution has several advantages such as accelerating the momentum of biomedical research, supplying high-level datasets, and supporting the authentication of research results. NIH presented a new Final NIH Policy for Data Management and Sharing which came into effect during January 2023. The new Policy requires NIH funded researchers to submit a plan that outlines how they will manage and share scientific data from their research. NIH-funded medical research has increased the life expectancy of United States citizens from forty-seven to seventy-eight years and the disability rate of citizens over the age of sixty-five years has decreased significantly.