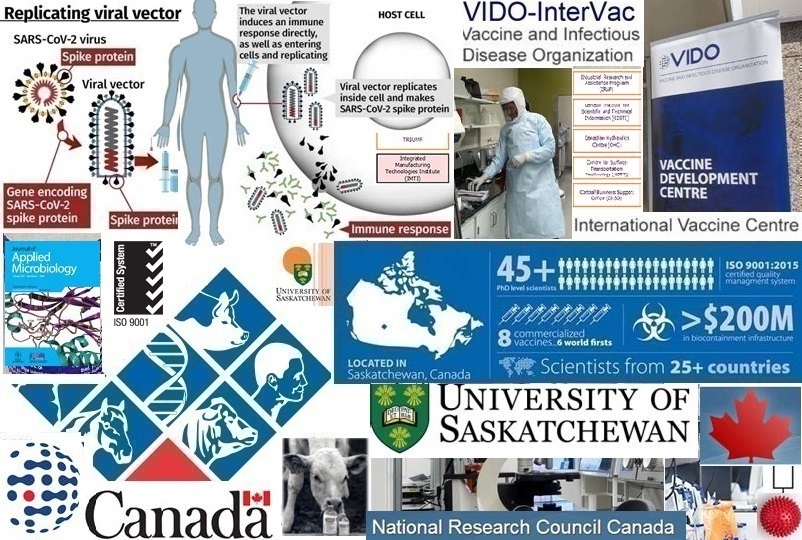
The Vaccine and Infectious Disease Organization – International Vaccine Centre (VIDO-InterVac) is a research establishment that operates from the University of Saskatchewan, Saskatoon, Canada, with financial support from the Government of Canada, the Government of Saskatchewan, livestock industrial agencies, councils, and foundations, as well as animal and human health companies Scientists at VIDO-InterVac have attempted to create a secondary-level vaccine production facility for the best part of ten years. Apart from operating as a research laboratory to protect Canada and the world from emerging and infectious diseases, they have also obtained an on-campus facility during this time. While laboratories around the world were active in trying to develop a vaccine, VIDO-InterVac identified an antigen to pass through the selective stages of testing for the possibility of a vaccine.
In the past, vaccine development by public laboratories in Ontario and Quebec made it possible to produce vaccines in Canada. During the 1950s, Toronto’s Connaught Laboratories played a major role in discovering the polio vaccine. It is the belief of Scott Halperin (Canadian professor of paediatrics, head of Paediatric Infectious Diseases at IWK Health Centre, immunologist, microbiology, and immunology at Dalhousie University (DU) in Halifax, and director of the Canadian Centre for Vaccinology at DU) that ‘vaccine independence is important, because relying on relationships with other countries to secure supplies of vaccines doesn’t always work.’ The long-term strategy is for Canadian researchers to continue developing coronavirus vaccines and therapeutics to establish a production line; thereby narrowing the gap to vaccine independence.
Health Canada is the department of the Government of Canada responsible for national health policies. The department manages several federal health-related agencies such as the Public Health Agency of Canada and the Canadian Food Inspection Agency to improve the economy, the environment, and the health of Canadian citizens by protecting animals, food, and plants. The latest threat was to detect avian influenza (AI) which is a viral infection identical to human influenza – it presents as a respiratory infection. AI (avian flu or bird flu) caused by influenza Type A viruses is like other animal flu caused by a virus strain adapted to a specific host. The category with the greatest risk to mankind is highly pathogenic avian influenza (HPAI). These viruses naturally spread globally among wild aquatic birds and infect domestic poultry and other bird and animal species. The Government of Canada funded research scientists at VIDO-InterVac to improve the influenza vaccine in Canada. The National Research Council of Canada (the primary national agency of the Government of Canada) is devoted to science, technology, research, and development. It is the largest federal research and development organisation in Canada. The Minister of Innovation, Science, and Economic Development is responsible for the NRC of Canada.
The Canadian Centre for Vaccinology (CCfV) at Dalhousie University in Halifax, Nova Scotia, is an integrated vaccine research team that researches, discovers, and evaluates vaccines; volunteers are chosen to take part in placebo-controlled studies. The Saskatchewan Health Authority (SHA) is the only health authority in Saskatchewan province. It provides direct health care and services such as, community and home care, primary, secondary, and tertiary care as well as mental health, addiction services, population and preventive health services. According to Dino Sophocleous, President and Chief Executive Officer of the Hospitals of Regina Foundation (HRF), the Foundation contributed to the new vaccine centre in their province by participating in a donation to VIDO to support the research and development of a made-in Saskatchewan COVID-19 vaccine and other vaccines that will positively impact the health of the southern Saskatchewan community, as well as to prepare for the next pandemic. The IWK Health Centre and Dalhousie University supports many talented researchers following projects that will ultimately lead to positive results for children, youth, women, and families. The Clinical Vision Science Program is an innovative graduate program of IWK Health Centre to improve professional clinical practice in ophthalmic and orthoptic medical technology. The importance is to understand the health conditions of the people.
VIDO has a strong bond with the Western College of Veterinary Medicine at the University of Saskatchewan. In March 2003, the laboratory adopted its present name, in October 2003, a five thousand square metre extension was completed, and in March 2004, VIDO received funds to build one of the world’s biggest and most sophisticated biosafety level 3 facilities. Funding of roughly one hundred and fifty million Canadian dollars was supplied for infrastructure by the Government of Canada, the Government of Saskatchewan, the ‘City of Saskatoon’, the Canada Foundation for Innovation, and the University of Saskatchewan. The 2011 completed level 3 facility known as the International Vaccine Centre (InterVac) can research emerging and re-emerging animal and human diseases with large animals such as alpacas, cattle, deer, elk, pigs, and sheep.
Canada’s National Research Council (NRC) is the largest national research and development organisation of the Government of Canada committed to science, technology, research, and development (the Minister of Innovation, Science, and Economic Development is responsible for the NRC). This organisation collaborates with Canadian industries to market research advantages to the people. Their motivation focuses on innovation to enhance people’s lives and address the world’s most demanding issues. VIDO-InterVac has created three offshoot companies namely Biostar, Biowest, and Star Biotech. In 2013, they received operational certification by Public Health Canada and the Canadian Food Inspection Agency. With one hundred and sixty personnel, more than two hundred and twenty-five million Canadian dollars in infrastructure, and forty-five years of experience, VIDO-InterVac is a global front-runner in infectious disease research and vaccine development.
VIDO-InterVac researchers are well-known for the development of animal models to study human diseases such as MERS-CoV, Zika, tuberculosis, respiratory syncytial virus (RSV), and pertussis. They were the first laboratory in Canada to establish an animal model to test antivirals and vaccines such as COVID-19 and the first laboratory in Canada to isolate the COVID-19 virus (SARS-CoV-2) from a clinical sample at Sunnybrook Health Sciences Centre in Toronto.
VIDO-InterVac has a record of vaccine development and marketing – eight VIDO vaccines have been sold commercially and six have been labelled as ‘global inventions.’ VIDO-InterVac took part in the world’s race to develop a coronavirus vaccine for COVID-19 by partnering with more than seventy organisations around the world to test COVID-19 treatments. The federal government assisted VIDO-InterVac with a fund boost of just over twenty-three million Canadian dollars. In April 2020, the COVID-19 Therapeutics Accelerator (CTA) partnered with VIDO-InterVac and awarded a grant of nearly eight hundred and thirty thousand Canadian dollars to expand its international status of testing antiviral compounds against COVID-19. The partnership was launched by the World Health Organisation (WHO) which is a specialised agency of the United Nations with six regional offices and one hundred and fifty field offices worldwide (headquartered in Geneva, Switzerland) responsible for international public health. CTA has supported the shortest, most synchronised, and successful global force in history to implement means of fighting COVID-19. With substantial improvements in research and development by academia, government initiatives, and the private sector, the ACT-Accelerator was the most innovative approach to move past the acute phase of the pandemic; applying the tests, treatments, and vaccines the world so desperately needed.
During the COVID pandemic, the Government of Saskatchewan provided just over four million Canadian dollars to VIDO-InterVac for clinical trials and operational expenses and twenty-three million Canadian dollars in federal funding to produce COVID-19 vaccines. InterVac obtained expertise through research on prior coronaviruses that spread to Canada during 2003 and 2012. Severe Acute Respiratory Syndrome (SARS) which was originally reported in Asia during February 2003 is a viral respiratory infection provoked by an airborne coronavirus known as SARS-associated coronavirus (SARS-CoV) that spreads through small saliva droplets in the same way as the cold and influenza. It was the first acute contagious disease of the 21st century that had the ability to spread via international air travel routes; therefore, it affected more than twenty-four countries in Asia, Europe, North America, and South America before the global outbreak could be contained. Canada (bordering North America) was also affected by SARS-CoV. Middle East Respiratory Syndrome (MERS) is a viral respiratory infection that is new to humans. It was initially reported in Saudi Arabia during 2012 and subsequently spread to various other countries including Canada. Most people infected with MERS-CoV experienced acute respiratory distress, cough, fever, and shortness of breath.
VIDO-InterVac identified a protein antigen that serves as a primary component of a vaccine contender against COVID-19. Approval was received from Health Canada to produce the antigen at laboratory scale. According to the Director and CEO of VIDO-InterVac, Dr.Volker Gerdts, the VIDO COVID-19 vaccine was developed with protein subunit technology which is an altered version of the spike protein of SARS-CoV-2 (the novel coronavirus that ‘instructs’ COVID-19) to arouse an immune response (offering protection against SARS-CoV-2 variants). The vaccine is cost effective, reliable, safe, and does not have to be stored at ultra-low temperatures. Continuous animal studies monitor any deviation in the effectiveness of the laboratory-scale antigen. VIDO-InterVac is the first non-governmental facility in Canada to research the African swine fever virus (ASFV) to develop a vaccine for ASF. According to the Canadian Food Inspection Agency (CFIA) ‘there are presently no approved vaccines to fight this pig disease. The research is a significant step towards the development and testing of antivirals and vaccines for African swine fever to protect Canada’s pork region.’ ASF is among the most troublesome animal disease pathogens. Although the pathogen does not pose a threat to humans, it affects the pork industry because pigs are a primary source of protein. The disease is a socio-economic risk and a burden to global food production and security. The ASFV is an infectious and complex DNA virus that only infects its natural hosts namely domestic pigs and wild boar. The virus causes a haemorrhagic disease with a mortality rate of mostly one hundred percent. ASF originated in Africa and spread to Eastern Europe and throughout China, resulting in the death of about forty percent of China’s swine. According to the World Organization for Animal Health, ASF affected over eight hundred and twenty-eight thousand pigs and above twenty-three thousand wild boars in forty-one countries since January 2021 – over and above one million animal fatalities!
Dr. Angela Rasmussen, BA, MSc, PhD. (Doctorate in microbiology) is a well-known research scientist, virologist, and adjunct professor at VIDO-InterVac, formerly an American-based research scientist, virologist, and faculty member at the Centre for Infection and Immunity at the Columbia Mailman School of Public Health in New York as well as the Georgetown Centre for Global Health Science and Security. She studied host responses to infection by combining classical virology methodologies with modern systems biology techniques to probe the host response to viral infection. Her research includes studying the interactions between hosts and pathogens and how they shape disease to distinguish between pathological emerging viruses that cause severe disease such as the ‘common cold’ (rhinovirus), Ebola virus, highly pathogenic avian influenza virus, and SARS-CoV-2 (COVID-19). Her research objectives are to recognise host responses that project infection severity or disease outcomes and host routes that suggest drug development or repurposing. She alters infections with highly pathogenic emerging viruses and studies different varieties of coronaviruses and how new strains can create distinctive activities, such as increased immunity. She informs that the COVID-19 vaccines are a significant means of defence, reduce the risks of developing the virus, and guard against critical disease. She is confident that VIDO is in the lead of Canadian research and innovation and that the third dosage will not only reduce serious disease and hospitalisation but will also fight Omicron. Although SARS-CoV-2 has troubled the nation, it has not been the deadliest pandemic of the last century.
All living organisms are made of cells that are the basic units of life responsible for the living and functioning of organisms. Cell biology studies functional and structural units of cells while molecular biology is the molecular foundation of biological action in and between cells such as the study of chemical and physical formation of biological macromolecules. Immunology is a division of biology and medicine that is the medical examination of immune systems in all organisms; therefore, we can see the dissimilarity of human immunology and related immunology in animal biosciences and veterinary medicine. Suitable animal models are crucial in evaluating vaccines. Virology is a subfield of microbiology and the scientific study of biological viruses. Viruses are infectious components that are loaded with genetic matter (DNA or RNA) that injects themselves into host cells. Epidemiologic studies show that viral infections in established countries are the mutual cause of severe ailments that does not require hospitalisation. In developing countries, viral illnesses cause permanent disability and a high mortality rate, particularly among children and infants. Developing viral illnesses such as infections due to Ebola virus, hantavirus, and HIV appear frequently. Its emphases are on the classification, detection, evolution, and structure of viruses, their means of infection and manipulation of host cells for replication, their interaction with host organism physiology and immunity, the illnesses they cause, the methods to separate and culture them, and their use in research and therapy. Virology includes all features of the virus – from evolution, function, life cycle, and structure to the sicknesses they cause as well as the host protections against them.
Orthohantavirus is a genus of RNA viruses that cause chronic asymptomatic infection in wild rodents, specifically mice and rats. Human infection with hantaviruses leads to a severe respiratory disease known as Hantavirus Pulmonary Syndrome (HPS) which can have fatal heart and lung complications. Invasion around or in the home remains the primary threat of hantavirus exposure. The virus is in the faeces, saliva, and urine of infected rodents. In restricted spaces it simply releases into the air. Ebola also known as Ebola Virus Disease (EVD) or Ebola Haemorrhagic Fever (EHF) is a rare but severe (often fatal) viral haemorrhagic fever caused by ebolaviruses in people and non-human primates. The virus is spread to people via direct contact with an infected animal, sick, or dead person that is infected with the Ebola virus. It can spread from person-to-person when they have close contact with infected blood, body fluids, diarrhoea, droplets, tissues, urine, or vomit. Ebola virus does not transmit through the air, water, or food; however, in certain regions of the world, Ebola virus can spread through touch and ingestion of wild animals infected with Ebola. Mosquitoes and other insects do not spread Ebola. The average EVD fatality rate is about fifty percent. Human Immunodeficiency Virus (HIV) is a virus that infects vital immune system cells and impairs their function (which is to fight infections), leaving the immune system exposed to other infections. The virus cannot be transmitted through sweat, urine, or saliva. Progressive destruction of the immune cells causes the infected person to become immunodeficient. The defective immune system eventually leads to the deadly disease Acquired Immune Deficiency Syndrome (AIDS). Scientists have not yet discovered a vaccine to fight either HIV or AIDS.
A fascinating facility at the University of Saskatchewan that is important to popular research is the Canadian Light Source Synchrotron which is one of the greatest science projects in Canadian history – delivering the sharpest light in the country (millions of times brighter than the sun). The Light Source stores twenty-two beamlines that supports a large selection of research applications. Each beamline presents a unique spectral range that provides independent information. Synchrotron science has vast benefits over conventional methods. The Canadian Light Source Synchrotron will be part two in the Canada series.